Abstract
Purpose
Treatment of prostate cancer with androgen deprivation therapy (ADT) is associated with an increased fat mass, decreased lean mass, increased fatigue and a reduction in quality of life (QoL). The aim of this study was to evaluate the efficacy of a 6-month dietary and physical activity intervention for prostate cancer patients receiving ADT, to help minimise these side effects.
Methods
Patients (n = 94) were recruited to this study if they were planned to receive ADT for prostate cancer for at least 6 months. Men randomised to the intervention arm received a dietary and exercise intervention, commensurate with UK healthy eating and physical activity recommendations. The primary outcome of interest was body composition; secondary outcomes included fatigue, QoL, functional capacity, stress and dietary change.
Results
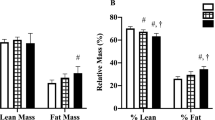
The intervention group had a significant (p < 0.001) reduction in weight, body mass index and percentage fat mass compared to the control group at 6 months; the between-group differences were −3.3 kg (95 % confidence interval (95 % CI) −4.5, −2.1), −1.1 kg/m2 (95 % CI −1.5, −0.7) and −2.1 % (95 % CI −2.8, −1.4), respectively, after adjustment for baseline values. The intervention resulted in improvements in functional capacity (p < 0.001) and dietary intakes but did not significantly impact fatigue, QoL or stress scores at endpoint.
Conclusions
A 6-month diet and physical activity intervention can minimise the adverse body composition changes associated with ADT.
Implications for Cancer Survivors
This study shows that a pragmatic lifestyle intervention is feasible and can have a positive impact on health behaviours and other key outcomes in men with prostate cancer receiving ADT.

Similar content being viewed by others
References
Hussain S, Gunell D, Donovan J, McPhail S, Hamdy F, Neal D, et al. Secular trends in prostate cancer mortality, morbidity, incidence and treatment: England and Wales, 1975–2004. BJU Int. 2008;101:547–55.
Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilisation of androgen deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer. 2001;92:2309–17.
National Institute for Health and Care Excellence (NICE). Prostate cancer—diagnosis and treatment. London: 2014
Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605.
Alibhai SMH, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–15.
Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–39.
Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603.
Galvao DA, Spry NA, Taffee DR, Newton RU, Stanley J, Shannon T, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–7.
Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with ADT. Clin Endocrinol. 2011;74:377–83.
Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. EJC. 2000;36:1134–41.
Herr HW, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163:1743–6.
Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, et al. Quality of life compared during pharmacological treatments and clinical monitoring for non-localised prostate cancer: a randomised controlled trial. BJU Int. 2004;93:975–9.
Alibhai SMH, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–45.
Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. JNCI. 2010;102:39–46.
Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9.
Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110:492–8.
Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–9.
Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–7.
Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer. 2010;18:591–9.
Department of Health UK. Chief Medical Officer’s report: evidence on the impact of physical activity and its relationship with health. London: HSMO; 2004.
Haskell WL, Lee I-M, Pate RR, Powell R, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendations for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sport Exerc. 2007;39:1423–34.
Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomised controlled trial. JAMA. 2009;301:1883–91.
Bourke L, Gilbert S, Hooper R, Steed LA, Joshi M, Jim WF, et al. Lifestyle changes for improving disease specific quality of life in sedentary men on long term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. 2013;65:865–72.
Haseen F, Murray LJ, O’Neill RF, O’Sullivan MJ, Cantwell MM. A randomised controlled trial to evaluate the efficacy of a 6 month dietary and physical activity intervention for prostate cancer patients receiving androgen deprivation therapy. Trials. 2010;11:86.
Durnin J, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97.
McCance & Widdowson’s ‘The composition of foods’. 5th edition B Holland, A A Welch, I D Unwin, D H Buss, AA Paul and DAT Southgate. The Royal Society of Chemistry, 1991.
Food Standards Agency: Eat well, be well. [http://www.food.gov.uk/].
Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3:211–6.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6.
Cella D, Eton DT, Lai J, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anaemia and fatigue scales. J Pain Symptom Manag. 2002;24:547–61.
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes in the Functional Assessment of Cancer Therapy-Prostate. Results from a clinical trial of patients with metastatic hormone refractory prostate cancer. Value Health. 2009;12:124–9.
Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials. 2000;21:167–89.
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials—the CONSORT statement. JAMA. 1996;276:637–9.
Galvão DA, Nosaka K, Taffe DR, Spry N, Kristjanson LJ, Mcguigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sport Exerc. 2006;38:2045–52.
Carmack-Taylor CL, Demoor C, Smith MA, Dunn AL, Basen-Enquist K, Nielsen I, et al. Active for life after cancer: a randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psycho-Oncology. 2006;15:847–62.
Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–83.
Isomma B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9.
Nobes JP, Langley SEM, Klopper T, Russell-Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012;109:1495–502.
Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50:918–23.
Culos-Reed SN, Robinson JL, Lau H, O’Connor K, Keats MR. Benefits of a physical activity intervention for men with prostate cancer. J Sport Exerc Psychol. 2007;29:118–27.
Bourke L, Doll H, Crank H, Daly A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers. 2011;20:647–57.
Stone P, Hardy J, Broadley J, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Brit J Cancer. 1999;79:1479–86.
Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, et al. Stages of change and decisional balance for 12 problem behaviours. Health Psychol. 1994;13:39–46.
Acknowledgments
Salary costs for this project were covered by the Department of Employment and Learning and the Centre of Excellence for Public Health, Queen’s University Belfast. The authors would like to acknowledge the contribution of the administrative and clinical staff at the Northern Ireland Cancer Centre, particularly nurses Barbara Harvey and Wendy McPhee. Special thanks also to the prostate cancer patients who gave so generously of their time.
Conflict of interest
Roisin F O’Neill, Farhana Haseen, Liam J Murray, Joe M O’Sullivan and Marie M Cantwell declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Neill, R.F., Haseen, F., Murray, L.J. et al. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J Cancer Surviv 9, 431–440 (2015). https://doi.org/10.1007/s11764-014-0417-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-014-0417-8